But less than 10 percent of tick species have been known to function as virus vectors ticks are vectors of arboviruses. Many tick-borne viruses (TBV) are RNA viruses a few of which cause severe diseases in people and creatures worldwide. Happen or TBV affecting domesticated or individual animal health are found to emerge recently. To be able to live in character, TBV should infect and reproduce in both the tick and vertebrate cells, representing surroundings that are different. Advice on mechanics that permit TBV to change between replicating and infecting from vertebrate and tick cells is more rare. Generally, ticks triumph in finishing their blood meal as a result of a multitude of biologically active molecules within their saliva which counteract and regulate various arms of their host defense reactions (haemostasis, inflammation, innate and acquired immunity, and wound healing). TBV's transmission can be and occurs through tick feeding. On the other hand, TBV transmission's underlying mechanisms are poorly known. Properties of tick saliva helping defeat the very first line of protection to premature and harm interactions in the skin port that is tick-host seem to be crucial in TBV transmission and disease of vertebrate hosts. The host epidermis type of tick attachment is a significant focal point of virus replication. Immunomodulation of this Tick Borno Virus attachment website also boosts co-feeding transmission of viruses from contaminated to non-infected ticks at the lack of server viraemia (non-viraemic transmission). Research ought to be directed toward identification of the tick salivary molecules boosting also a description of interactions, along with also virus transmission and also of course epidermis immunomodulation. Such advice will permit the reason style of vaccines which protect against infection brought on by viruses.
Intro
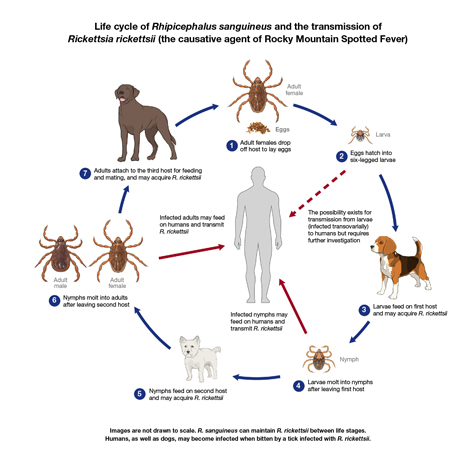
Ticks are recognizable to people. Humans have been accompanied by them through their history, known. But because the pioneering work of Smith and Kilbourne about Texas fever of cattle followed by Rickett's finding of this pathogen causing Rocky Mountain spotted fever transmitted by Dermacentor andersoni at 1907, ticks are identified as vectors of an massive variety of viral, viral, and protozoan agents of ailments, and have come to be a significant focus of health and clinical study (Nicholson et al., 2009; Sonenshine and Roe, 2014). It is understood that ticks transcend the other arthropods from the assortment of infectious agents between nematodes, fungi, viruses, protozoa, bacteria, and viruses. Tick-borne viral ailments have increasing and significant health and behavioural effect on account of the geographic spread of their vectors and outbreaks from new areas as a consequence of fluctuations in global climatic and socio-economic conditions, and absence of efficient management steps (Estrada-Peña et al., 2013; Nuttall, 2014; Vayssier-Taussat et al., 2015; Brackney and Armstrong, 2016). Herewe believe potential directions for study, the explanations for ticks are productive and efficient vectors of viruses, along with the viruses transmitted by ticks.
Tick-Borne Viruses
Tick-borne viruses (TBV) or even"tiboviruses" (Hubálek along with Rudolf, 2012) include a diverse set of viruses circulating involving pollutants and vertebrate hosts, yet flourishing in two different surroundings: the homeostatic surroundings of their vertebrate host and the radically changing surroundings of airways. Infection and TBV have emerged causing a relationship where the virus life cycle is coordinated with the feeding cycle of the tick, without impacting its own biology along with also the tick could harbor the virus for extended periods.
The first discoveries of arboviruses like yellow fever virus (1928) (mosquito-borne), also Nairobi sheep disease virus (1910) along with louping ill virus (LIV) (1929) (tick-borne)and opened the floodgates to its discovery of over 500 arboviruses throughout the years (Bichaud et al., 2014). At least 160 termed viruses have been tick-borne, of which approximately 50 are known or likely"virus species" (Nuttall, 2014). Taxonomically, TBV include a heterogenous set of vertebrate viruses categorized into a DNA viral household, Asfarviridae, also eight RNA viral households: Flaviviridae, Orthomyxoviridae, Reoviridae, Rhabdoviridae, the recently known Nyamiviridae (sequence Mononegavirales), along with the households Nairoviridae, Phenuiviridae, along with Peribunyaviridae from the new arrangement, Bunyavirales (Table 1).

Classification of RNA viruses such as newly described species, together with hint of viruses causing diseases of animals and humans.
The incidence of TBV in various viral families indicates their tick-borne manner of transmitting evolved separately at least seven days (Nuttall, 2014). 25% of TBV are connected with illness and TBV pathogenic to people are zoonotic. Many TBV cause severe human or animal ailments, like CNS infection (meningitis, meningoencephalitis, or encephalomyelitis), or haemorrhagic disorder (Table 1). Others tend to be reported or severe, and many are without known veterinary or medical significance. Particular viral ailments of feral vertebrates in addition to domestic animals and even individuals might pass unnoticed or are misdiagnosed, and they might seem as emerging disorders (Dörrbecker et al., 2010; Hubálek and Rudolf, 2012).
Recently, a range of established TBV, chiefly those belonging to this tick-borne encephalitis virus (TBEV) serocomplex, have emerged or re-emerged, or disperse, posing a growing threat to animal and human health. By way of instance, a increase in the prevalence of infections brought on by Powassan virus (POWV) from the USA (Hermance and Thangamani, 2017), the spread of TBEV into new geographical locations, along with the development of new viruses like Alkhurma virus, also a subtype of both Kyasanur forest disease virus (KFDV) (Charrel et al., 2001), along with Deer Tick Borne virus, also a subtype of both POWV (Pugliese et al., 2007; Robertson et al., 2009; Hermance and Thangamani, 2017) are reported. The newest emerging TBD, brought on by Bourbon virus (Thogoto virus, also Orthomyxoviridae), has been reported in Kansas at 2014 (Kosoy et al., 2015).
While fresh TBV happen to be found, unclassified viruses have been allocated to both genera or families because of developments in molecular technology (Table 1). The most noteworthy changes have happened in households Bunyaviridae along with Rhabdoviridae. TBVs are comprised in three households --Nairoviridae, Phenuiviridae, along with Peribunyaviridae. Lately, a new virus strongly linked to SFTSV called Heartland virus had been isolated from seriously febrile patients from the USA (McMullan et al., 2012) and out of area collected ticks (Savage et al., 2013). Additionally, phylogenetic and serological investigations demonstrated that Bhanja virus and Palma virus (formerly unassigned into some genus) are closely associated with both SFTVS along with Heartland virus (Dilcher et al., 2012; Matsuno et al., 2013).
In the last several decades, additional novel rhabdoviruses are identified from several animal species, however thus far transmission by ticks are confirmed just for some of these, e.g., Kolente virus (Ghedin et al., 2013) along with Yongjia signal virus two (YTV-2) (Li et al., 2015).
Recent research indicate that apart from the hitherto only known DNA comprising arbovirus, African swine fever virus (ASFV) (Asfarviridae, genus Asfivirus), conducted by soft ticksalong with additional DNA viruses could be transmitted by ticks. Could it be the emerging virus disease's origin?






